Case ID:
HJF 508-18
Web Published:
1/27/2023
Researchers at The Henry M. Jackson Foundation (HJF), The Beth Israel Deaconess Medical Center (BIDMC), the Armed Forces Research Institute of Medical Sciences (AFRIMS), and the Walter Reed Army Institute of Research (WRAIR) have identified several monoclonal antibodies (mAbs) with a wide-range of capabilities to precisely neutralize Zika virus (ZIKV) or potently cross-neutralize Zika and Dengue virus (DENV). Potential applications of these antibodies include use in diagnostics, vaccines, clinical and diagnostic assays and as antibody-based research tools.
Applications and Advantages
- Precise: Offers option of mAbs capable of potent, specific ZIKV or ZIKV and DENV cross- neutralization without reactivity to other flaviviruses
- Refined: Demonstrated conformational binding to Zika and Dengue virus particles and protection against Zika and Dengue, In vivo
- Pragmatic: Reduces risk of contracting Zika virus and subsequent complications in global/international travelers with no prior flavivirus exposure
- Widespread Impact: Advances prevention, diagnostic and treatment efforts for flaviviruses in humans
Innovation Description
ZIKV is an emerging pandemic disease, particularly in Central and South America. Most often transmitted by mosquitoes, ZIKV infection can lead to congenital and neurological complications such as microcephaly and Guillain-Barre syndrome. ZIKV infections typically occur in travelers with no prior exposure to a flavivirus- the genus of RNA viruses that include dengue, West Nile, and yellow fever.
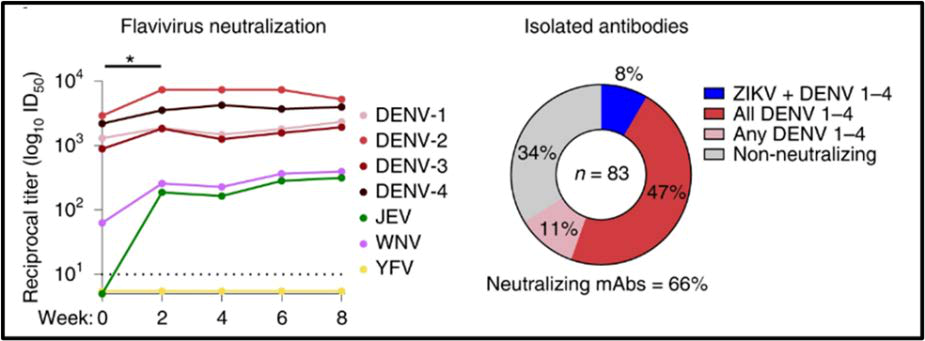
Fig 1. ZPIV vaccination boosts pre-existing immunity and elicits cross-neutralizing antibodies in a flavivirus-experienced individual
Currently there are no approved treatments for ZIKV or DEVZ infections, underlining the need for approved preventative and therapeutic options. Researchers at HJF and collaborators have identified several mAbs with a wide-range of capabilities to precisely neutralize ZIKV or potentially cross-neutralize ZIKV and DENV. These novel mAbs can be used alone or as antibody fragments for the prevention and treatments of ZIKV and DENV infections, even in individuals with no prior flavivirus exposure such as global/international travelers. Further applications of these mAbs treatments include use in diagnostics, vaccine manufacturing, clinical and diagnostic assays and as antibody-based research tools.
Inventors
- Shelly J. Krebs, Ph.D., HJF
- Kayvon Modjarrad, M.D., Ph.D., HJF
- Michael Joyce Gordon, Ph.D., HJF
- Gina C. Donofrio, M.S., HJF
- Vincent Dussupt, Ph.D., HJF
- Dan Barouch, M.D., Ph.D., BIDMC
- COL (Ret) Nelson Michael, M.D., WRAIR
- CAPT Richard Jarman, Ph.D., AFRIMS
Innovation Status
Currently, pK and toxicity studies are being planned. Please see: Nat Med 2020 Feb 03; 26:228
Intellectual Property Status
Patent applications have been filed in Canada (3,098,373), Singapore (10202251412D), India (202017050993), and Brazil (1120200219282). A U.S. application has been allowed (17/049,800).