Case ID:
HJF 383-14
Web Published:
1/13/2023
A technology related to novel therapeutics for treating graft-versus-host disease (GvHD) is available for licensing from HJF. In vivo animal studies have shown its efficacy in reducing infiltrating inflammatory T cells in the colon as well as reduction of GvHD-associated weight loss and mortality.
Applications and Advantages
- Novel protein therapeutics for GvHD
- Significant efficacy demonstrated in animal models
- Comparable efficacy to rapamycin but with lower risks of side effects and toxicity
Innovation Description
Acute graft-versus-host disease (aGVHD) is an immune-mediated reaction that can occur after hematopoietic stem cell transplantation in which donor T cells recognize the host antigens as foreign, destroying host tissues. Establishment of a tolerogenic immune environment while preserving the immune response to infectious agents is required for successful bone marrow transplantation. Pregnancy-specific glycoprotein 1 (PSG1), which is secreted by the human placenta into the maternal circulation throughout pregnancy, likely plays a role in maintaining immunotolerance to prevent rejection of the fetus by the maternal immune system. Scientists from the Uniformed Services University of Health Sciences (USUHS) and the National Institues of Health (NIH) have shown that PSG1 activates transforming growth factor β (TGF-β), a cytokine essential for the differentiation of tolerance-inducing regulatory T cells (Tregs). Furthermore, treatment of naive murine T cells with PSG1 resulted in a significant increase in Tregs that was blocked by a TGF- β receptor 1 inhibitor and PSG1 can increase the availability of active TGF- β in vivo. As the role of Tregs in the prevention of aGVHD is well established, the scientists tested whether PSG1 has beneficial effects in a murine aGHVD transplantation model. PSG1-treated mice had reduced numbers of tissue infiltrating inflammatory CD3+ T cells and had increased expression of FoxP3 in T cells compared with vehicle treated mice. In addition, administration of PSG1 significantly inhibited aGVHD-associated weight loss and mortality.
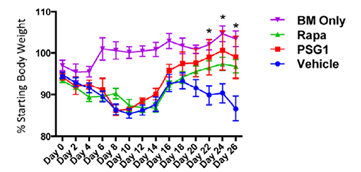
Figure 1. An extended course of PSG1 in acute GVHD increases FoxP3 expression, decreases weight loss, and induces comparable or improved outcomes compared with treatment with rapamycin. Mice treated with an extended course(26 days) of PSG1 showed marked improvement in weight gain over vehicle-treated mice. This weight gain was comparable to that observed in mice receiving rapamycin. As it is observed in the short duration treatment (14 days), there were marked differences in clinical scores and survival between the mice treated with vehicle (scores of 4 to 5) and PSG1 treated mice (scores of 0 to 1)(P = .003).
Inventors
- Gabriela Dveksler, Ph.D. USUHS
- Harry Malech, M.D. NIH
Innovation Status
In vivo efficacy is shown in animal studies. Please see: Biol Blood Marrow Transplant. 2019,25(2):193-203.
Intellectual Property Status
Patents have been issued in the U.S. (11,007,249), Canada (2,981,191), U.K. (3261722), and Australia (2016323769).