Case ID:
HJF 474-17
Web Published:
12/1/2022
A novel composition of malaria vaccine technology is available for licensing from HJF. A Phase I clinical trial has been completed and results have shown the safety and efficacy of this vaccine composition in humans.
Applications and Advantages
- Novel immunogens and compositions for malaria vaccine
- Significant clinical efficacy demonstrated in humans
- Fully characterized and patented adjuvant
Innovation Description
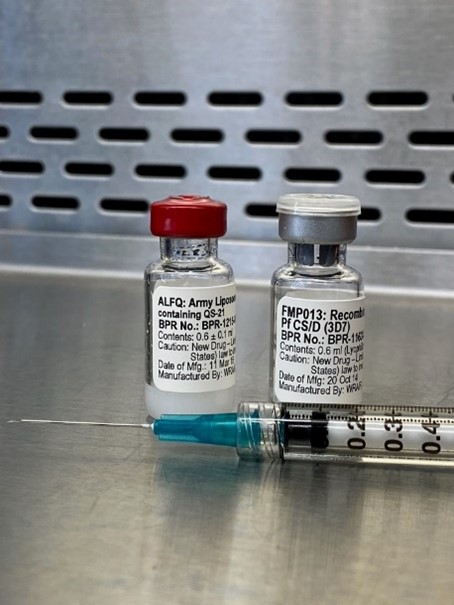
Malaria, caused by the parasite Plasmodium falciparum, kills hundreds of thousands of children annually. Emergence of multi-drug-resistant strains indicates an urgent need for an efficacious vaccine for those living in malaria-endemic areas, as well as for those who travel to these areas such as tourists and troops.
Scientists from the Walter Reed Army Institute of Research (WRAIR) and HJF and have developed a novel malaria vaccine composition that contains a novel Plasmodium falciparum circumsporozoite protein (CSP) immunogen FMP013 formulated with the patented adjuvant ALFQ. FMP013/ALFQ had high immunogenicity in animals including mice and rhesus macaques and was selected for further clinical studies in humans.
The results from the Phase I clinical study have shown that the FMP013/ALFQ composition is safe in humans and offers significant protection against parasitemia in subjects who received controlled human malaria infection. In addition, serological profiling revealed that humans who received FMP013/ALFQ developed high-titer CSP antibody responses.
Inventors
- Sheetij Dutta, Ph.D. WRAIR
- Zoltan Beck, Ph.D. HJF/WRAIR
- Carl Alving, Ph.D. WRAIR
- Gary Matyas, Ph.D. WRAIR
Innovation Status
Phase I clinical study has been completed. Please see: Clinicaltrials.gov and Vaccine 2019. 37:3793-3803 .
Intellectual Property Status
A patent application is allowed in the U.S. (16/607,917), and others are pending in Europe (18791678.8) and India (201917046420).