Case ID:
HJF 630-22
Web Published:
2/7/2024
Henry M. Jackson Foundation (HJF) researchers have developed a novel vaccine strategy against HIV-1. These vaccine candidates are designed to elicit broadly neutralizing antibodies that could protect against a wide variety of HIV strains.
Applications and Advantages
- Novel vaccine design approach that emulates the diversity observed in HIV-1 infections with multiple founder variants
- Vaccine candidates that correspond to a cocktail of novel nucleic acid and protein sequences adapted to any HIV-1 subtypes and circulating recombinant forms
- Expected to promote broadly neutralizing antibodies (bnAbs) response and block infection
Innovation Description
The immense global diversity of HIV-1 is a significant obstacle to developing a safe and effective vaccine. To counteract HIV-1 diversity, there is a need of a vaccine that could elicit broadly neutralizing antibodies (bnAbs) to cross-react with a variety of HIV-1 envelope (Env) glycoproteins. BnAbs have the potential to neutralize a wide variety of HIV strains and affect the course of HIV infection by directly engaging host immunity.
HJF researchers analyzed viral sequences sampled during acute HIV-1 infection to the subsequent breadth of immune responses in participants enrolled in a prospective acute infection cohort conducted by U.S. Military HIV Research Program (MHRP). They showed that HIV neutralization breadth can be shaped during early HIV infection. Half of the individuals who developed bnAbs acquired infections with multiple HIV founder variants, whereas all individuals with limited neutralization breadth had been infected with single HIV founders.
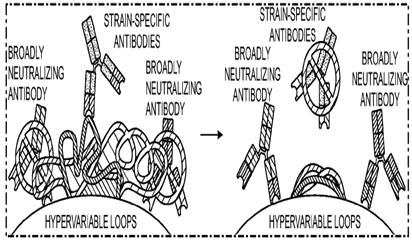
From these findings, Drs. Lewitus, Bai and Rolland proposed a novel vaccine strategy (Fig. 1) utilizing minimally distant multiple founder-like HIV immunogens, which are more closely related than any other multivalent vaccine strategy, to best elicit broad protective immune responses found in natural infection. Immunization with a cocktail of closely-related antigen variants initiates a cross-reactive process leading to the maturation of broadly neutralizing antibodies.
Fig. 1 Schematic path to the elicitation of broadly neutralizing antibodies
with multiple founder variants.
Inventors
- Morgane Rolland, Ph.D., HJF
- Eric Lewitus, Ph.D., HJF
- Hongjun Bai, Ph.D., HJF
Innovation Status
In silico data available. Please see PLoS (Pathog) 2020 Feb 6;16(2):e1008179 ; PLoS (Comput Biol) 2022 Oct 31;18(10):e1010624 .
Intellectual Property Status
A PCT patent application on the subject invention has been filed (PCT/US2023/065187).