Case ID:
HJF 637-22
Web Published:
10/12/2023
Greater than 20% of traumatic wound closure attempts fail due to the immuno-molecular status of the healing wound tissue.
Researchers at HJF and the Uniformed Services University for Health Sciences (USU) developed a product that analyzes the individual's wound healing immune response and computes a risk of failure score based on cytokine levels within the wound effluent. The algorithm and diagnostic kit can be packaged and sold to healthcare facilities to minimize wound failure following surgical closure.
Applications and Advantages
- A novel prognostic tool that quantifies the probability of successful wound closure – no other tool is commercially available
- Informed predictive model based on correlation differences among twelve biomarkers across healed and dehisced wounds
- Clinical Decision Support Tool (CDST) to assist surgeons identify wounds that are ready for closure reducing the risk of adverse events
Innovation Description
Dehiscence (a partial or total separation of previously approximated wound edges), due to a failure of proper wound healing has been reported to occur 15-30% in combat-related and civilian trauma wounds. The ability to effectively discriminate between wounds that are likely to proceed through normal vs. delayed wound healing is important for preventing worsening dehiscence, infection, and other complications. Hence, a surgeon’s decision regarding the timing of closure of large, traumatic extremity wounds is of high clinical significance for positive patient outcomes.
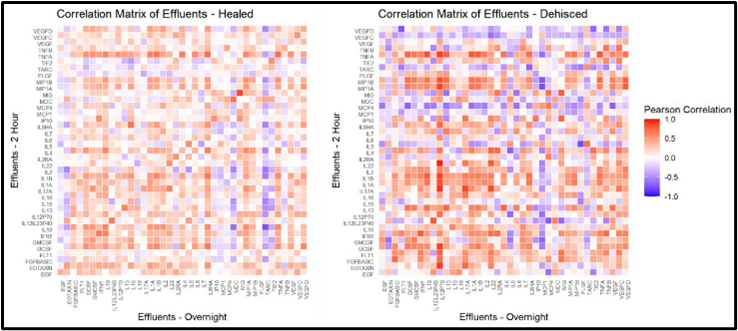 Researchers at HJF and USU have developed WounDx™, a Clinical Decision Support Tool (CDST) based on correlations among the biomarkers across healed and dehisced wounds (Fig.1). The tool provides a surgeon with wound-specific probability that an open wound will heal normally if closed. WounDx consists of two components: an assay kit and predictive model. A subset of clinical characteristics and effluent biomarkers taken before, during, and after debridement were used to develop the current prediction model. Studies included a cohort of combat casualties treated at the Walter Reed Military Medical Center (WRNMMC) and a cohort of civilian acute trauma patients at Grady Memorial Hospital in Atlanta, Georgia.
Researchers at HJF and USU have developed WounDx™, a Clinical Decision Support Tool (CDST) based on correlations among the biomarkers across healed and dehisced wounds (Fig.1). The tool provides a surgeon with wound-specific probability that an open wound will heal normally if closed. WounDx consists of two components: an assay kit and predictive model. A subset of clinical characteristics and effluent biomarkers taken before, during, and after debridement were used to develop the current prediction model. Studies included a cohort of combat casualties treated at the Walter Reed Military Medical Center (WRNMMC) and a cohort of civilian acute trauma patients at Grady Memorial Hospital in Atlanta, Georgia.
Fig. 1 Correlation matrix of overnight and two-hour post debridement
effluent biomarkers by successfully healed/closed and dehisced wounds.
Inventors
- CAPT. Eric Elster, M.D., USU
- Seth Schobel-McHugh , Ph.D., HJF
- Henry T. Robertson, Ph.D., HJF
- Felipe Assis Lisboa, M.D., HJF
- Michael Rouse, Ph.D., HJF
- Scott F. Grey, Ph.D., HJF
Innovation Status
A simulation of 380 wounds were followed over multiple debridements. Data from current clinical practice wound closure rates (for comparison to current practice) and estimated wound closure rates using the final prediction model were created for numerous thresholds.
Intellectual Property Status
A PCT patent application has been filed.