Case ID:
HJF 616-21
Web Published:
12/12/2024
Researchers at HJF, Inova Fairfax Hospital (IFH), and the Peter MacCallum Cancer Center (PMCC) have identified orthogonal protein and transcript-based biomarkers correlating with Homologous Recombination Deficiency (HRD). This may lead to a companion clinical diagnostic assay to prioritize High-Grade Serous Ovarian Cancer (HGSOC) patients for targeted DNA damage response therapies.
Applications and Advantages
- Development of an expression-based assay that identifies tumor tissue as HR proficient (HRP) or deficient (HRD)
- Companion diagnostic assay for stratifying HGSOC patient tumors based on HR status
Innovation Description
HGSOC is one of the most malignant diseases that affect women, accounting for 70-80% of ovarian cancer deaths. HGSOC patients can harbor tumors with mutations in genes that respond to DNA damage. This can lead to impaired DNA repair, causing HRD. A class of therapeutics, including poly ADP ribose polymerase (PARP) inhibitors, have been developed to specifically target HGSOC patients with tumors classified as HRD. Currently, four PARP inhibitors have been approved by the FDA for treating cancers of the ovary, breast, prostate, and pancreas classified as HRD. Companion diagnostics approved for identifying HRD-cancers focus on detecting alterations in DNA sequences that are consistent with impaired homologous recombination DNA repair. There is an unmet need for diagnostic methods that can inform on the status of HR and its functional state of activity in HGSOC tumor cells.
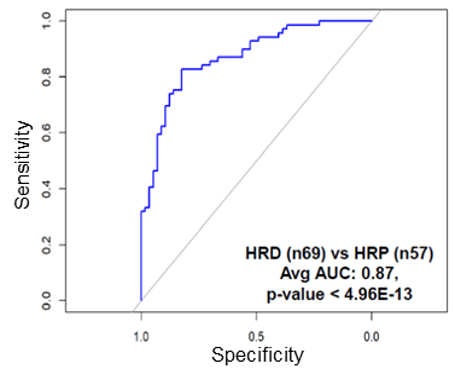
To address this need, our researchers have validated expression (protein and transcript) alterations from multi-omic analysis of HGSOC tissues stratified by HR status. They have identified an optimized panel that includes 11 biomarker candidates that exhibit superior performance to classify HGSOC patient tumors as HRD or HRP (Fig. 1). Such analysis of HRD-correlated protein and transcript alterations in tumor biopsies may function as a companion clinical diagnostic assay to prioritize patients for targeted DNA damage response therapies such as treatment with PARP inhibitors.
Fig.1 Optimized 11-panel biomarker performance
Inventors
- Nicholas Bateman, Ph.D. HJF
- Tamara Abulez, MS., HJF
- Thomas Conrad, Ph.D. IFH
- George L. Maxwell, M.D., IFH
- David Bowtell, Ph.D. PMCC
- Dale Garsed, Ph.D., PMCC
- Ahwan Pandey, MS., PMCC
Innovation Status
Candidate biomarkers have been identified in a set of HGSOC tumor samples with complete germline and somatic mutation annotation by whole-genome sequencing, transcriptome and proteome data. The biomarker panels are currently being refined and validated using independent cohorts. Please see Nat Genet. 2022 Dec;54(12):1853
Intellectual Property Status
A PCT patent application has been filed